Introduction
Severe acute respiratory syndrome corona-virus 2 (SARS-CoV-2), the causative agent of COVID-19, has led to a global pandemic, affecting 290 million people and 5.4 million deaths till 7th January 2022. To contain this brunt, various novel vaccines received an Emergency Use Authorization (EUA) by the U.S.A Food Drug Administration (FDA), European Medicine Agency (EMA), U.K. Medicines and Healthcare Products Regulatory Agency (MHRA) and Indian Central Drugs Standard Control Organization (CDSCO) as well as Drugs Controller General of India (DCGI).1 About 910 million people are administered with vaccine doses. These vaccines are administered to health-care workers, front-line workers, elderly individuals including those with comorbidities. Since health care workers are at the front line of the ongoing pandemic, they were the first to receive the vaccines, thus, evaluating the antibody response aids to render a snapshot of the burden of SARS-CoV-2 infection amid HCWs.2 Various studies on immune response for SARS-CoV-2 showed that antibodies were present 7-14 days after the onset of symptoms.3, 4, 5 These antibodies were found to decline with the passage of time but remain detectable for several months following infection.6, 7 It was observed that SARS-CoV-2 antibodies have neutralizing capacity in-vitro and shield against reinfection in study models.8 Till today, early data has been published on immunogenicity and safety of the vaccine, however, associated factors such as duration of immune response and rate of antibody production is yet to uncover.9, 10 Therefore, the present study aimed to investigate the production of total antibodies against SARS-CoV-2 before and after vaccination (2 doses) among HCWs along with associated factors such as co-morbidities and adverse reaction after vaccination.
Materials and Methods
The present study was conducted on 60 healthcare workers (34 males and 26 females within the age group of 22-64 years) as cases who got vaccinated for COVID-19 vaccine at Government Medical College and Hospital, Amritsar. Before commencement of the study, ethical permission and approval was obtained from the Ethics Commission at Viral Research and Diagnostic Laboratory (VRDL), Government Medical College (GMC), Amritsar, India. Individuals vaccinated with COVID-19 vaccine were enrolled as cases while individuals with previous SARS-CoV-2 history and/or were symptomatic at the time of sample collection were excluded. About 5ml of blood sample was withdrawn to detect COVID-19 specific IgG Ab base line titre. The first blood samples were taken at day 0 before vaccination followed by 4th week, 8th week, 12th week, 24th week post vaccination. The semi-quantitative detection of anti-SARS-CoV-2 IgG Ab in the samples was detected using ErbaLisa COVID-19 IgG indirect ELISA kit using standard protocol as described by manufacture. Antibody titre was evaluated on the basis of cut off values following comparison between negative control and sample. The sample was evaluated negative if its value was similar to the negative control whereas if the value obtained was at-least 2 times higher than cut off value of negative control it was considered as positive.
Results
The present study was conducted on 60 health care workers vaccinated for COVID-19 at Government Medical College, Amritsar. Co-morbidities such as hypertension was presented in only 8 (12.3%) individuals, diabetes in 4 (6.1%) and coronary artery disease in 1 (1.5%). After vaccination, 56% of individuals experienced weakness, 79% had fever while malaise, joint pains and body aches were reported in 33.33%, 3.33% and 16.67% subjects, respectively.
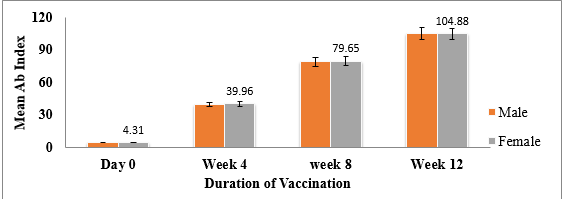
In this study, after first dose of administration, 86.67% HCWs were found seropositive for the anti-SARS-CoV-2 Abs while 13.33% were seronegative, however, after second dose, 100% seroprevalence was observed amid HCWs (Table 1). The statistical analysis depicted a significant difference (p<0.0001) in antibody titres among individuals before vaccination and after vaccination. The mean of total antibody levels at zero week (4.1), 4th week, (39.09), 8th week (79.39) and 12th week (105). Overall, no statistically significant difference was observed between antibody response, gender and age (Figure 1, Figure 2).
Table 1
Total antibody index among health care workers before and after vaccination
n-66; Day Zero:- Total Ab Index before Vaccination (p Value-); Day 28:- Total Ab index after 1st Dose 1st sampling (p Value-); Day 56:- Total Ab index after 2nd dose 1st sampling (p Value-); Day 84:- Total Ab index after 2nd Dose 2nd sampling (p-value < 0.05)
Discussion
Vaccination plays a dynamic role in reducing the COVID-19 pandemic. The key question that needs to be addressed here is that how much protection does vaccines provide in controlling the infection by producing more antibodies. ChAdOx1 nCoV -19 (AZD1222) vaccine has been found to induce Anti-spike antibody, antibody-dependent monocyte/neutrophil phagocytosis, and cellular responses.11
In this study, total antibody levels were measured by ELISA for comparing the results before and after vaccination among healthcare workers. Significantly higher levels of antibodies were observed after first and second dose of vaccination as compared to prior vaccination among all healthcare workers (p<0.0001). A study conducted on 515 individuals who were administered both the doses of vaccination showed that 95.0% were observed to be seropositive for the anti-SARS-CoV-2 antibodies. 12 A comparative study on HCW and non-HCW demonstrated no significant difference in seroprevalence among health care workers.13 Zhang et al., 202114 observed higher Ab measurements in subjects who had SARS-CoV-2 infection followed by 2 doses of vaccination as compared to individuals who got vaccinated without prior infection. A cohort study on 2607 subjects also observed significantly higher antibody levels (p<0.001) after vaccination. 15 A meta-analysis of 49 studie showed overall seroprevalence of 8.7% among HCWs. 16 A previous study performed at Oxford University Hospital reported that seroprevalence of SARS-CoV-2 antibodies after first dose in participants was 94% within 15-21 days and 97% in 22-28 days.17
Our findings suggest that there is no correlation between Ab response, gender and age. A study by Uysal et al., 2021. 18 and Singh et al., 2021 12 showed similar findings indicating no relationship between age and antibody levels. However, Kataria et al., 2021 13 found that age had significant impact on Ab responses which was less in individuals with age >60 years (30.6%) as compared to age ≤ 60 years (46.4%). Study by Salvong et al., 2021 19 showed that age and gender were independent predictors of antibody levels. Few other studies have reported gender differences in response to COVID-19 vaccinations. 20, 21, 22, 23 These differences in correlation among Ab response, gender and age can be attributed to variation in population demographics in different regions or countries depicting weaker or strong immune responses in individuals of different age groups.
In our study, only 12.3% individuals had hypertension, 6.1% had diabetes and 1.5% suffered from CAD, however, none of these co-morbidities had any impact on Ab titre. Singh et al., 2021, demonstrated that people with type 2 diabetes showed less seropositivity rate in comparison to those without T2D (p<0.001) after completion of both doses. Post vaccination effects were seen in many cases and the most common being weakness followed by fever, malaise, joint pains and body aches in the present study. Other studies indicated mild to moderate side effects post vaccination. 12 Local and systemic adverse reactions, including injection site pain, chills, muscle ache, and headache, have also been reported by Folegetti et al., 2020.24 According to Sasso et al., 2021,15 1184 out of 2607 subjects displayed one or more adverse reactions after vaccination which includes muscular pain and systemic effects. Jeong et al., 2021 25 observed that adverse reactions were significantly associated with positivity rate and these variables may be used as parameters to estimate the formation of anti-SARS-CoV-2 antibodies.
Conclusion
This study renders paramount information on the detectable levels of antibodies induced by ChAdOx1 nCOV-19 against the SARS-CoV-2 after first dose and second dose in the population of Punjab. Robust immunity induced by the above said vaccine against COVID-19 measured by total IgG ELISA was also reported previously.24 Since the vaccines for SARS-CoV-2 have been recently developed, questions pertaining to the durability of protection over the period after vaccination and determination of effect of booster dose to extend the duration of immunity for COVID-19, are yet to be answered. Thus, it is important to understand that serological testing is important prior to vaccination to analyse the humoral immune response of the subject, so that the vaccination protocol could be adapted. This approach could accelerate vaccination regimes, facing new variants, achieving immunity quickly, stopping the spread and hindering emergence of new variants.