- Visibility 35 Views
- Downloads 3 Downloads
- DOI 10.18231/j.agems.2022.017
-
CrossMark
- Citation
Diurnal variation of peak expiratory flow rate in adolescents
- Author Details:
-
Spandana Charles *
-
Archana
-
Padmavathy
-
Priscilla
-
Subhashini
Introduction
Circadian rhythm is a roughly 24 hour cycle in the biochemical, physiological or behavioral processes of living entities. These rhythms are endogenous but can be adjusted to the environment by external cues called zeitgebers. The primary circadian "clock" in humans is located in the suprachiasmatic nucleus of the hypothalamus.[1], [2] Entrainment of the circadian clock is by the classical photoreceptors and the ganglion cells in the retina which convey information about illumination to the SCN through the retino hypothalamic tract. It has been observed that core body temperature,[3] pulse rate,[4] renal function,[5] gastric acid secretion,[6] lung functions[7] as well as secretion of hormones like cortisol, thyrotropin, growth hormone and serotonin[8] exhibit a circadian rhythm. Chronobiology is particularly important in the lung since a circadian rhythm in airway resistance is easily demonstrated and the implications of this rhythm are relevant to the diagnosis of asthma, and to the design and interpretation of trials of bronchodilator drugs. Peak expiratory flow rate (PEFR) is a simple and reliable way of assessing lung function.[9] It is an easily measured and reproducible parameter for quantifying airway obstruction and has a positive correlation with forced expiratory volume in first second (FEV1),[10], [11] Hence; this parameter is chosen for monitoring PEFR on a longitudinal basis which is a valuable and reliable tool in surveys of respiratory health in adolescents. Long and short term daily PEFR monitoring can provide the subject and clinician with objective data upon which therapeutic decisions can be made. This may also aid in timing medical treatment in coordination with PEFR variability which may significantly increase efficacy and reduce drug toxicity or adverse reactions. Limited information is available on the diurnal variation of lung function in Indian healthy subjects. Hence, the aim of this study is to determine the diurnal rhythm in indices of pulmonary function variability during the usual daytime hours in a population of normal adolescents.
Materials and Methods
Selection and Description of Participants: This longitudinal study was conducted among 30 adolescent girls in the age group of 18-19 years after obtaining informed consent from study subjects and Institutional ethical committee clearance. Subjects with history of smoking, Bronchial asthma, Tuberculosis, recent surgeries and pregnancy were excluded.
Technical information
Information on demographic, socioeconomic, nutritional, sleep pattern, and exposure to indoor and outdoor pollution were collected using a study questionnaire. General examination and detailed respiratory system examination was performed and the clinical examination findings were noted in the Performa. Standing height in centimeters was measured using a measuring tape, Weight was recorded in Kilograms using a portable weighing machine. Waist, chest and arm circumferences were measured using a measuring tape. Body mass Index (BMI) was calculated by using the formula: Weight (in Kg)/Ht in meter2.
PEFR was measured using the Mini — Wright s (AIRMED, Clement Clarke International limited), peak flow meter. The subjects were individually trained for measuring their PEFR in L/min. This procedure is repeated 2 – 3 times and the best reading is recorded. If the subject coughs or does not perform the procedure correctly, the reading is repeated after taking a break of 15 to 30 minutes.
Data collection
PEFR was measured four times a day (5.30 – 6 .00 am, 12.00 – 1.00 pm, 5.00 - 6.00 pm, 9.00 – 10 .00pm) for a period of 3 months to study the diurnal variation of lung function. These values were recorded by each subject in their own diary which was provided to them.
Statistical Analysis
Diurnal variation for each subject was calculated using amplitude % mean (Highest PEF-Lowest PEF)*100/Mean PEF and standard deviation % mean (SD of PEF values)*100/mean PEF (Higgins et al. 1997)12. The PEFR values at different time points were analyzed using analysis of variance.
Results
This study was done on 30 adolescent girls living in Chirala a coastal town in Andhra Pradesh located 100 km south of Vijayawada. The subjects had a mean age of 18.9 with the minimum age being 18 and the maximum 19. The mean height was 154cm (minimum 143cm, maximum 171cm) and the mean weight was 46.5kg (minimum 31kg, maximum 60kg).The mean BMI was 19.57( minimum 15.2, maximum 27).
Since PEFR varies with age, sex, height and weight we have selected female subjects of nearly the same age, height and weight. Hence, the inter-individual variations in the study population due to age, sex, height and weight have been minimized. Out of the 30 subjects selected for the study 24 completed the study. Hence, the lost to follow up percentage is 20%. Details of the descriptive (anthropometric) parameters of the study subjects have been provided in [Table 1].
|
Parameter |
Mean |
Median |
SD |
Minimum |
Maximum |
|
Height |
154.1 |
154 |
6.7 |
143 |
171 |
|
Weight |
46.6 |
45.5 |
7.2 |
31 |
60 |
|
BMI |
19.6 |
19.3 |
2.5 |
15 |
27 |
|
Waist |
68.5 |
67 |
8.2 |
56 |
90 |
|
Chest |
79.3 |
79.5 |
6.9 |
65 |
95 |
|
Midarm |
24.5 |
24 |
2.2 |
21 |
29 |
Comparison of mean PEFR
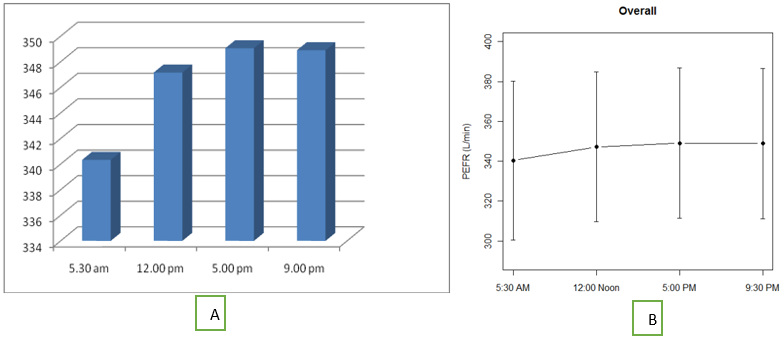
The mean PEFR recorded at 5.30 am, 12.00 noon, 5.00 pm and 9.00 pm were 340, 347, 349 and 348 respectively. [Figure 1] a and 1b show mean PEFR at these points in time. The results suggest that PEFR in these subjects exhibits a definite circadian rhythm, it being lowest early in the morning, progressively rises till late afternoon following which there is a slight fall in the night. This pattern was observed in 88% of subjects. The difference in PEFR between the four different points in time when compared using repeated measures ANOVA was found to be statistically significant (p value<0.001). Holm-Bonferroni test was done to compare the morning and evening PEFR and the difference was statistically significant.
Amplitude % mean
Diurnal variability in peak flow was calculated using amplitude percent mean.
Formula used for calculating amplitude % mean is:
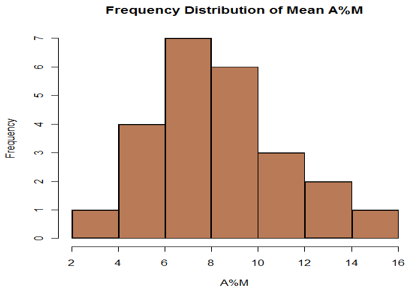
Highest PEF-Lowest PEF *100 (Higgins et al 1997)Mean PEFThe mean of amplitude % mean obtained in this study using was 8.42 ± 3.65. [Figure 2] shows the frequency distribution of amplitude % mean. All the subjects had an amplitude % mean of less than 20. The 95% confidence limit for the upper limits of amplitude percent mean was 14.2.
Standard deviation % mean
Standard deviation % mean was also calculated for diurnal variability.
The formula used for calculating the standard deviation % mean was:
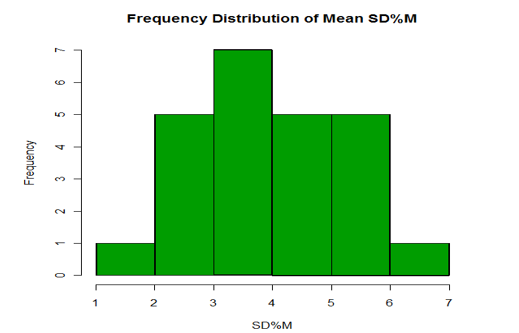
SD of PEF values*100Mean PEFThe standard deviation % mean obtained in this study was 3.87±1. [Figure 3] depicts the frequency distribution of standard deviation % mean.
.
Discussion
The present study has evaluated diurnal variation of PEFR among adolescent girls by collecting PEFR data, four times in a day, for a period of three months using Wright’s peak flow meter. The results indicate presence of significant diurnal variation in PEFR among healthy adolescent girls. Similar results have been observed in studies from India and other countries.
Methodological Strengths of this study: PEFR is known to vary with age, gender, nutritional status, differences in body composition etc. Inter individual variation in PEFR due to age, gender, anthropometry, nutritional factors and environmental pollutants has been substantially minimized in this study as this study was conducted only in girls and differences in body composition was minimal. All the study participants were living in hostel, hence inter individual differences due the exposure to air pollutants was also minimal. Out of the 30 recruited for this study, 24 participants completed the total time-consuming protocol, indicating good compliance among the participants. The study participants have judiciously performed the PEFR measurements every day continuously for a 30-day period each month for three months under the supervision of the hostel warden after getting properly trained by the investigator.
PEFR measurement using Wright’s peak flow meter is a simple, economical and reproducible test, and can be easily done by the participants with appropriate training than the challenge tests. PEFR correlates well with bronchial hyperactivity and is an accepted marker of caliber of the airways. Collection of PEFR data for one or two days for calculation of PEFR variability can result in training effect as PEFR is effort dependent parameter. In this study PEFR data was collected for a period three months, four times a day for assessment of the PEF variability, thereby reducing the variability caused by effort based factors.
Studies on PEFR variability are limited in India and none from this geographical location among adolescent population. This is the first ever study conducted in healthy adolescent girl population examining the diurnal variation of PEFR. The mean PEFR obtained in our study for adolescent girls in the age group of 18-19 yrs with a mean height of 154 cm and a mean weight of 46.5 kg was 346.3 L/min.
Diurnal variation in PEFR was observed in these study participants. The lowest PEFR was observed in the morning. The diurnal variation in lung function was estimated by comparing the mean PEFR across different time points (5.30am, 12.00 noon, 5.00 pm, and 9.30pm). The results of this study showed that PEFR in normal subjects exhibited a definite circadian rhythm characterized by lowest values in the morning followed by a progressive rise peaking in the evening and a small fall at bed time. The results of this study showed that PEFR in normal subjects exhibited a definite circadian rhythm characterized by lowest values in the morning followed by a progressive rise peaking in the evening and a small fall at bed time. The results are similar with the results of Higgins, Shahbaz afzal et al.,[12] Aggarwal et al. 2000,[13] Jindal et al. 2002,[14] Goyal et al. 2008[15] and Richa et al. 2010.[16]
PEF variability can also be measured using amplitude % mean because of its statistical performance and simplicity of its clinical use as calculated earlier by Higgins and colleagues (Higgins et al. 1997. Diurnal variability expressed as amplitude % mean was calculated for these subjects using 4 values was 8.42±3.65. The amplitude % mean observed in this study was similar to the values observed in other studies as shown in [Table 2].
|
Author |
Population & Geographical location |
Amplitude% mean |
|
Agarwal et 2000 |
Adults 20-40 years(men and women) Chandigarh |
7.23±3.48 |
|
Richa thaman 2010 |
Elderly males 60-80 yrs Punjab |
10.69±4.58 |
|
Shahbaz Afzal |
Adult males 18-26 yrs Lahore |
4.11% |
|
Higgins 1997 |
Adults 18-65 ( men and women) England |
8.5 vs 9.7% |
|
Goyal 2008 |
Young males(18-26) Lucknow |
7.55±4.1 |
|
Sapaldia study 1999 |
Adults 18-60 yrs(men and women) Switzerland |
5.2% |
|
Ranzi et al 2004 |
Children 6-11 yrs Italy |
6.7±1.6 |
|
Present study |
Adolescent girls 18-19 yrs Andhra Pradesh |
8.42±3.65 |
Agarwal et al. measured seven readings of PEFR a day among young male volunteers and observed an amplitude % mean of 7.23 ±3.48. Richa Thaman et al. on took 5 readings of PEFR a day on Geriatric subjects and showed a mean diurnal variation of 10.69 ± 4.58 (A% M). Studies done by Shahbaz Afzal et al. using 5 readings a day showed an amplitude percent mean of 4.11% in healthy men. Mean diurnal variation, as observed by Goyal et al. on young men in the age group of 18-25, was 7.55±4.1 (A%M). Higgins et al. took 2 hourly readings during the waking hours and observed an amplitude % mean of 9.7% in women and 8.5 % in men. In the Sapaldia [17] study the mean PEF variability calculated using 2 readings a day in women was 5.2% and in men it was 5.6%. In this study an amplitude % mean of 8.42±3.65 was observed when 4 readings were considered. It has been observed by Higgins that PEFR variability was more in women when compared to men, this could be the reason the values of this study are slightly more than those observed by Agarwal, Shahbaz and Goyal. Lebowitz and coworkers have shown that in normal subjects, PEFR variability increases with an increase in the number of PEFR readings used to derive PEFR variability. A minimum of 4 readings would be preferred to calculate the amplitude % mean or the variability may be underestimated especially in asthmatics.
The standard deviation% mean obtained in our study was 3.87± 1.6(Fig 4). The SD % mean obtained in other studies are: Agarwal et al.(2.98 ±1.31), Richa et al. 4.36 ± 1.90 (SD% M), Goyal et al. 2.79±1.42. Hence, it is almost similar to the observations of other studies.
The 95% upper confidence limit observed in our study was 14.2. Agarwal et al. 2000 established upper limits of normal variability at 14.31 and 16.31 at 95% and 99 % confidence limits. The upper limits of normal variability A% M established by Richa et al. 2010 at 95% and 99% confidence limits were 12.04 and 12.46, respectively. Goyal et al. observed the upper limits of normal variability A%M at 95% and 99% confidence limits to be 15.75 and 19.85, respectively. Diurnal variation of more than 20% is observed in asthmatics and this criteria is now being used to diagnose remission of bronchial asthma (Hetzel et al. 1980).
Relevance
In conclusion the study has provided the data which can be used as baseline for studying the trend of PEFR, PEFR variability (PEFRvar) its relation with several factors such as body composition among the young population in future. PEFRvar measurement protocol followed in this study can be used in large scale epidemiological studies for screening, diagnosis, assessment of severity of disease and prognosis of respiratory disease especially bronchial asthma.
Limitations of the study
Though the technique of measuring PEFR using the Mini- Wright s peak flow meter was taught and demonstrated to the study subjects the readings were not taken under supervision, Sample size is small, Study was done only on female subjects.
Abbreviations List
PEFR: Peak expiratory Flow Rate: The greatest rate of airflow that can be obtained during forced exhalation, which follows a diurnal pattern of fluctuation and can be used clinically to evaluate airway tone.
FEV1: Forced expiratory volume in first second: It is the maximal amount of air that can be forcefully exhaled in one second.
BMI: Body Mass Index: A measurement of the relative percentages of fat and muscle mass in the human body, in which mass in kilograms is divided by height in meters squared and the result used as an index of obesity.
A%M: Amplitude Percent Mean: Defined as the ratio between difference between the maximal and minimal PEF and the mean PEF as a percentage.
SD%M: Standard Deviation Percent Mean: Defined as the ratio between the standard deviation of PEf values and the mean PEF as a percentage.
PEFRvar: Peak Expiratory Flow Rate Variability: Defined as the variability of the peak expiratory flow rate.
Author Contributions
Dr. Archana. P. Kumar: Has made substantial contributions to acquisition of data and analysis of data, has drafted the submitted article and has provided final approval of the version to be published.
Dr. R. Padmavathi: Has made substantial contributions to conception and design of the study and interpretation of data; has revised the article critically for important intellectual content and has provided final approval of the version to be published.
Dr. Priscilla Johnson: Has made substantial contributions to conception and design, or acquisition of data; has drafted the submitted article and has provided final approval of the version to be published.
Dr. Subhashini: Has made substantial contributions to conception and design, has revised it critically for important intellectual content; and has provided final approval of the version to be published.
Source of Funding
None.
Conflict of Interest
None.
References
- SJ Aton, CS Colwell, AJ Harmar, J Waschek, ED Herzog. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 2005. [Google Scholar]
- AC Liu, DK Welsh, CH Ko, HG Tran, EE Zhang, AA Priest. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007. [Google Scholar]
- WP Colquhoun, RS Edwards. Circadian rhythms of body temperature in shift-workers at a coalface. Br J Ind Med 1970. [Google Scholar]
- G Cornelissen, F Halberg, HW Wendt, C Bingham, RB Sothern, E Haus. Resonance of about-weekly human heart rate rhythm with solar activity change. Biologia (Bratisl). 1996. [Google Scholar]
- DS Minors. Circadian rhythms of urinary excretion: the relationship between the amount excreted and the circadian changes. J Physiol 1982. [Google Scholar] [Crossref]
- JG Moore, E Englert, H Brown. Circadian rhythm of gastric acid secretion in man. Clin Res 1961. [Google Scholar]
- Z Bendova, A Sumova. Photoperiodic regulation of PER1 and PER2 protein expression in rat peripheral tissues. Physiol Res 2006. [Google Scholar]
- M Hastings, SO’ John, ES Neill. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol 2007. [Google Scholar]
- M Goval, A Goel, P Kumar, M Bajpai, NS Verma, S Kant. Circadian rhythm of peak expiratory flow rates in healthy north Indian men. Indian J Physiol Pharmacol 2008. [Google Scholar]
- JN Pande, A Mohan, S Khilani, GC Khilani. Peak expiratory flow rate in school going children. Indian J Chest Dis Allied Sci 1997. [Google Scholar]
- G Rosenblatt, I Alkaly, P Mccann, M Stein. The correlation of peak flow rate with maximal expiratory flow rate, one second forced expiratory volume and mximum breathing capacity. Amer Rev Resp Dis 1963. [Google Scholar] [Crossref]
- S Ashfaq, M Saeed. Diurnal Variation in Peak Expiratory Flow Rates of Healthy Young Adults. Saudi J Med Pharm Sci 2000. [Google Scholar]
- AN Aggarwal, D Gupta, S Chaganti, SK Jindal. Diurnal variation in peak expiratory flow in healthy young adults. Indian J Chest Dis Allied Sci 2000. [Google Scholar]
- SK Jindal, AN Aggarwal, D Gupta. Diurnal variability of peak expiratory flow. J Asthma 2002. [Google Scholar]
- M Goval, A Goel, P Kumar, M Bajpai, NS Verma, S Kant. Circadian rhythm of peak expiratory flow rates in healthy north Indian men. Indian J Physiol Pharmacol 2008. [Google Scholar]
- RG Thaman, K Kawalinder, P Geeti, Girgla. Circadian Peak Expiratory Flow Rate Variability in Healthy North Indian Geriatric Population. JIACM 2010. [Google Scholar]
- N Künzli, EZ Stutz, AP Perruchoud, O Brändli, JM Tschopp, G Bolognini. Peak flow variability in the SAPALDIA study and its validity in screening for asthma-related conditions. The SPALDIA Team. Am J Respir Crit Care Med 1999. [Google Scholar]
