- Visibility 53 Views
- Downloads 9 Downloads
- DOI 10.18231/j.agems.2022.013
-
CrossMark
- Citation
Possible menstrual cycle changes after COVID 19 vaccination a questionnaire-based study among vaccinated women
Introduction
The seventh human coronavirus, SARS-CoV-2, was found in Wuhan, Hubei Province, China, during a recent pneumonia epidemic in January 2020. Since then, the virus has spread all over the world, infecting 665,339,248 people and killing 6,698,470 people as of January 2nd, 2023. SARS-CoV-2, SARS-CoV, and the Middle East respiratory syndrome coronavirus (MERS-CoV) all-cause severe pneumonia, with death rates of 2.9 percent, 9.6 percent, and 36%, respectively. OC43, NL63, HKU1, and 229E are the other four human coronaviruses that induce self-limited sickness with modest symptoms.[1] Coronaviruses are enclosed RNA viruses with a crown-like shape that can range in size from 60 nm to 140 nm in diameter and are prevalent in animals, including humans and birds. Coronaviruses have been found to mutate and recombine, resulting in diseases of the respiratory, gastrointestinal, hepatic, and nervous systems. Coronavirus includes seven strains, including HKU1, NL63, 229E, and OC43, SARS-CoV, MERS-CoV, and SARS-CoV-19 (COVID-19 being the most recent), the first four of which produced moderate respiratory sickness in infected humans, while the other three caused human death. Previously, in the years 2002–03, SARS affected almost 8000 people, with 774 of them dying. In 2012, 2494 people were infected and over 858 people died as a result of MERS-CoV, and COVID-19 has caused 5,56,335 deaths in 216 countries (as of 11th July 2020). Because of its mutation and recombination properties, the genomic sequence of SARS-CoV-2 has changed since it was first described.[2] COVID-19 infected 1 lakh people in 67 days (March 7th), another 1 lakh in 12 days (March 19th), and a third invasion took only 4 days (March 23rd), showing that SARS-CoV-2 is a highly transmissible type virus. By the end of March, the global number of confirmed cases had risen to 7.25 lakhs. After H1N1 (2009), polio (2014), Ebola (2014 in West Africa), Zika (2016), and Ebola (2019 in the Democratic Republic of Congo), the COVID-19 outbreak is the sixth PHEIC (public health emergency of international concern).[3] The potential severity of the disease, even in young persons without comorbidities, is a key contrast between COVID-19 and seasonal influenza-associated pneumonia. In a study that compared three well-conducted Chinese case series to a reference group of patients with influenza-associated pneumonia from 73 German sentinel hospitals, the severity of the pneumonia was significantly higher in COVID-19, even in people aged 60 years without chronic preconditions. For example, no comorbidity was observed in 28% of COVID-19 patients treated in the ICU. COVID-19 patients had a significantly greater rate of acute respiratory distress syndrome (ARDS) and mechanical ventilation.[4] Fever, cough, weariness, and myalgia were the most common symptoms at the outset of sickness across all investigations. According to current data, only half of the patients are febrile at the time of admission.[5], [6] Anorexia, nausea, vomiting, and diarrhea are all common gastrointestinal complaints, with nearly 40% of patients reporting them in certain studies. Additionally, up to 10% of individuals report gastrointestinal symptoms but no respiratory or fever symptoms. COVID-19 has been linked to an elevated risk of venous thromboembolism due to a hypercoagulable condition. Muscle injury, ischemic and hemorrhagic strokes, and neurological symptoms (including headache, dizziness, and altered consciousness) have also been recorded. In a small Italian cohort, a third of patients experienced taste or olfactory problems, such as anosmia. Skin and visual signs are examples of other extrapulmonary manifestations. A research in Italy found that 20% of individuals had cutaneous symptoms. In a Chinese case series, 32 percent of COVID patients had ocular symptoms compatible with conjunctivitis.[7]
Vaccines of Covid 19
As of December 2nd 2022, 11 vaccines were granted Emergency Use Listing (EUL) by WHO. Live attenuated, inactivated, protein subunit, virus-like particles, virus vectored (nonreplicating and replicating: with or without antigen-presenting cell), mRNA, and DNA are among the ten vaccine possibilities for COVID-19. Any of the structural proteins (Spike (S), membrane (M), Envelope (E), and viral RNA genome), as well as non-structural proteins (nsp 1e16) and nine accessory proteins, could be vaccine targets.[8]
Covishield and Covaxin
On January 21st, India granted emergency use authorization to Covishield and Covaxin. Covishield is a non-replicating adenovirus vaccine, while Covaxin is a viral inactivating vaccination. In preclinical investigations, both Covishield and Covaxin showed encouraging outcomes. Covishield (ChAdOx1 nCoV-19) has shown both humoral and antibody responses in phase I and II trials, as well as a good safety profile. 18 In the interim analysis, the continuing phase three trial was also demonstrated to have an excellent safety profile and efficacy (70.4 percent). In a study of 130 confirmed cases, COVAXIN showed 77.8% vaccination effectiveness against symptomatic COVID-19 disease, with 24 cases in the vaccine group against 106 in the placebo group. 93.4 percent effectiveness against severe symptomatic COVID-19 disease has been demonstrated. The effectiveness studies show that it protects against asymptomatic COVID-19 by 63.6 percent.[9] From 15 Jan 2021 to till 15 NOV 2021 total 1,12,34,30,478 vaccination doses[10] are administered including Covishield and Covaxin.
Sputnik
The results of the study demonstrate a robust protective impact across all age categories of participants. The vaccine, also known as Gam-COVID-Vac, uses a heterologous recombinant adenovirus method to express the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein utilizing adenovirus 26 (Ad26) and adenovirus 5 (Ad5) as vectors. The use of two different serotypes, each given 21 days apart, is designed to circumvent any population-wide viral immunity.[11]
Covid Vaccination and Menstrual Cycle
A sore arm, fever, and myalgia are all common side effects of covid-19 immunization, according to the UK's Medicines and Healthcare Products Regulatory Agency (MHRA). Although changes in menstruation and sudden vaginal bleeding is not noted, patients who have encountered these events quickly after vaccination are increasingly approaching primary care providers and reproductive health professionals. By 2 September 2021, more than 30 000 reports of these incidents have been made to the MHRA's yellow card surveillance scheme for adverse medication reactions, spanning all covid-19 vaccines now available.[12] People who report a change to their period after vaccination find it returns to normal in the following cycle. Covid-19 vaccination has not been shown to negatively affect fertility in clinical trials. In study after study, unintended pregnancies occurred at similar rates in vaccinated and unvaccinated groups.[13] Women have reported experiencing menstrual changes after receiving mRNA and adenovirus vectored vaccines for covid-191, suggesting that the link may be the result of an immune response to vaccination rather than a specific vaccine component.[14] There are also associations between vaccination against human papillomavirus (HPV) and changes in the menstrual cycle.[15] Certain stimuli, including viral infection, can cause immune activation, which can cause changes in the menstrual cycle. While definitive data is still awaited, how should professionals advise patients who have experienced these side effects in the meantime? Initially, they should be urged to contact the MHRA's yellow card scheme if their periods vary or if they experience sudden vaginal bleeding. This will provide more comprehensive data to aid study into any potential link, as well as convey to patients that their concerns regarding vaccine safety are taken seriously, thereby fostering trust. The Royal College of Obstetricians and Gynecologists and the Medicines and Healthcare Products Regulatory Agency (MHRA) recommend that anyone reporting a change in periods that lasts many cycles or new vaginal bleeding after menopause be treated. The usual clinical guidelines should be followed for these conditions when it comes to treating menopause.[16] To determine whether their menstrual cycle changed following covid vaccination, a questionnaire was prepared and distributed to the vaccinated women.
Materials and Methods
This was a cross-sectional study. The study population included 106 women aged between 18 to 52 years. Data was collected through a self-administered Questionnaire which was shared with eligible participants through Google Forms after obtaining their Informed Consent. Any woman of reproductive age who was still in the pre-menopausal stage was considered eligible to participate in this study.
Data collection
The women enrolled in this study were provided with a self-administered pre-validated questionnaire in the form of a Google Form with a brief explanation of the objectives of the study. Participation in the study was voluntary. A participant's completion and return of the questionnaire was considered informed consent for participation in the study. No personal data, including names, addresses, etc. were collected. The questionnaire was based on a literature review in this field. And suitably modified to suit the current scenario.[17] The questionnaire comprised 9 questions, and participants were able to choose the best option based on the multiple options provided. Participants were also given the option to comment on their own experiences with the normal menstruation cycle and after receiving the Covid 19 vaccination. They were asked about their own menstrual cycle experiences after the Covid 19 vaccination, their vaccination history, the name of the vaccine their experiences after the first and second doses of vaccination.
Data analysis
Data was collected from the completed questionnaires, and all the information was entered into an Excel sheet for preliminary analysis and final writing. The demographic profile of women and the experience of women concerning the effects of vaccines on menstrual cycles were used as independent variables. The results are expressed as percentages.
Results
We collected responses from 106 women for the study. Of those, 104 women were able to answer all the questions that were required by he study.
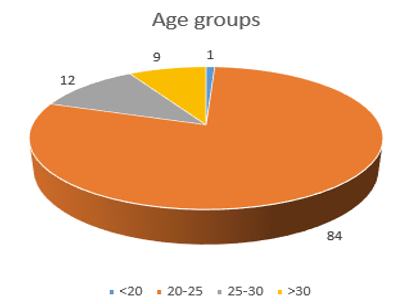
In [Figure 1], the age distribution of the total subjects shows that 1 participant belongs to the 18-20 years group, 84 belong to the 20-25 years group, 12 belong to the 25-30 years group, and 9 Participants belong to the 30-50 years group.
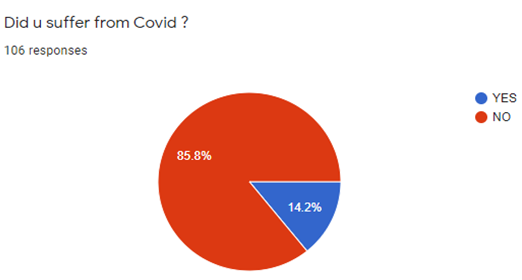
As shown in [Figure 2], out of 106 responses, 15 participants (14.2%) reported they suffer from COVID 19, while 91 participants (85.7%) stated they do not suffer from COVID 19.
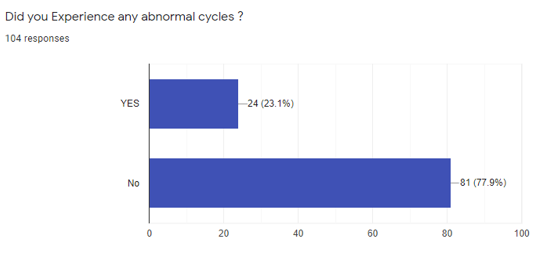
[Figure 3] illustrates the distribution of abnormal menstrual cycles following vaccination. In the 104 Participants, 24 participants (23.1%) reported abnormal menstrual cycles, and 81 participants (77.9%) didn't have abnormal menstrual cycles.
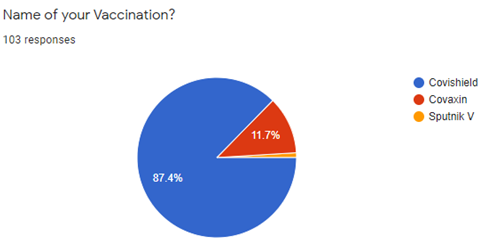
[Figure 4] shows that out of the 103 Participants who answered this question 90 (90.7%) said they took Covishield, 12 (90.7%) took Covaxin, while 1 (11.7%) took Sputnik V.
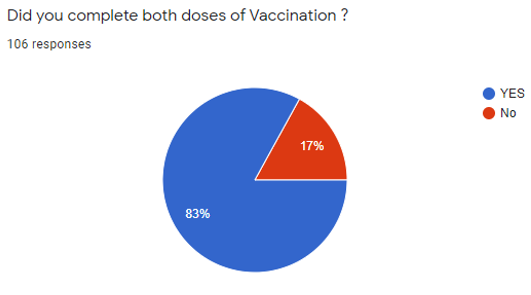
[Figure 5] shows that in total 106 participants, 88 (83%) went through both vaccination doses, and 18 (17%) received the single dose.
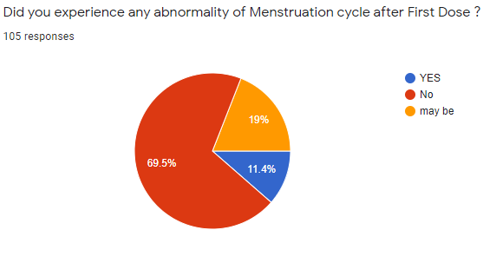
[Figure 6] shows that in the 105 Participants, 73 participants (69.5%) indicated NO, 12 participants (11.4%) indicated YES, and 20 participants (19%) indicated they are not sure.
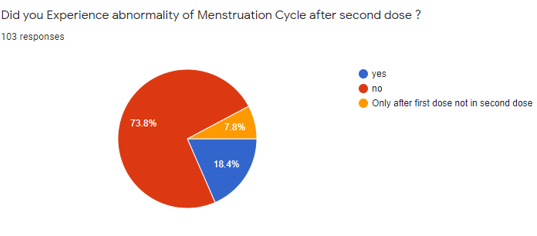
The above [Figure 7] shows that of the 103 Participants, 76 participants (73.8%) stated No and 19 participants (18.4%) said Yes, and 8 individuals (7.8%) said they experienced menstrual irregularities only after the first dosing.
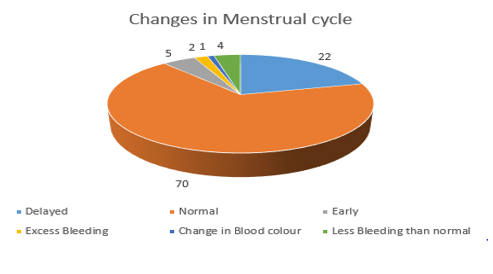
[Figure 8] Out of 104 Participants, 70 Participants (67.3%) reported they did not have any changes, 22 Participants (21.1%) reported delayed cycles, 5 Participants (4.8%) reported early cycles, and 4 Participants 3.8 claimed they had less bleeding than normal. One participant (0.9%) reported that their blood color changed.
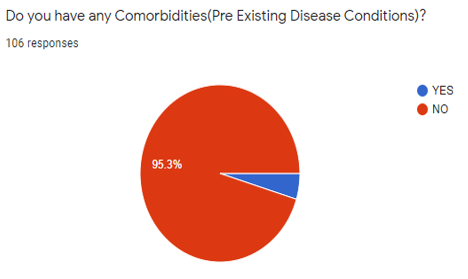
[Figure 9] shows that, out of 106 participants, 101 said no 95.3% said no 5 said yes (4.7%).
Discussion
Adenovirus or mRNA vectored covid-19 vaccines have been associated with menstrual changes, suggesting that, if a relationship exists, it will most likely be because of the immune response rather than from a specific component of the vaccine In addition to immunological influences on hormones driving the menstrual cycle, there may be biological mechanisms to explain immune stimulation and menstrual changes. A study of the possible association between covid-19 vaccines and menstrual changes may also shed light on the mechanism behind this phenomenon Despite the short-lived effects of vaccination on the menstrual cycle, research into this possible adverse reaction is essential to the overall success of the vaccination program.[18] Still, we do not have conclusive proof that vaccinations are responsible for changes in the menstrual cycle, but more research will provide more clear answers.
Conclusion
There were 24 women (23.1%) who reported abnormal menstruation cycles out of the 104 participants, and 81 women (77.9%) did not have abnormal menstruation cycles. This does not support the hypothesis that vaccinations might cause abnormal menstruation cycles. However, women experienced changes in their cycle, and that cannot be denied. Future studies on the relationship between vaccinations and the menstrual cycle based on larger data may yield more clarity.
Source of Funding
None.
Conflict of Interest
None.
References
- M Ciotti, M Ciccozzi, A Terrinoni, WCJiang. The COVID-19 pandemic. Crit Rev Clin Lab Sci 2020. [Google Scholar]
- R Ghosh, S Nundy, K Tapas. How India is dealing with COVID-19 pandemic. Sensors International 2020. [Google Scholar] [Crossref]
- I Chakrabort, P Maity. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ 2020. [Google Scholar] [Crossref]
- M Cevik, CGG Bamford, A Ho. COVID-19 pandemic-a focused review for clinicians. Clin Microbiol Infect 2020. [Google Scholar]
- K Tolksdorf, S Buda, E Schuler, LH Wieler, W Haas. Influenza-associated pneumonia as reference to assess seriousness of coronavirus disease (COVID-19). Eurosurveillance 2020. [Google Scholar] [Crossref]
- M Cevik, CGG Bamford, A Ho. Clinical Microbiology and Infection. Clin Microbiol Infect 2020. [Google Scholar]
- SK Kaushik, S Bobdey, DS Faujdar, V Anand. Coronavirus disease 2019 vaccines: perspectives and update. Med J Armed Forces India 2021. [Google Scholar]
- L Dai, GF Gao. Viral targets for vaccines against COVID-19. Nat Rev Immunol 2021. [Google Scholar]
- . COVAXIN® - India's First Indigenous COVID-19 Vaccine. . [Google Scholar]
- . IndiaFightsCorona COVID-19. 2023. [Google Scholar]
- DY Logunov, IV Dolzhikova, DV Shcheblyakov. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021. [Google Scholar]
- . Medicines and Healthcare Products Regulatory Agency. Coronavirus vaccine-weekly summary of yellow card reporting. 2021. [Google Scholar]
- V Male. Are covid-19 vaccines safe in pregnancy?. Nat Rev Immunol 2021. [Google Scholar]
- . Medicines and Healthcare Products Regulatory Agency. Coronavirus vaccine-weekly summary of yellow card reporting. 2021. [Google Scholar]
- S Suzuki, A Hosono. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res 2018. [Google Scholar]
- V Male. Menstrual changes after covid-19 vaccination. BMJ 2021. [Google Scholar] [Crossref]
- İ Esen, B Oğuz, HM Serin. Menstrual Characteristics of Pubertal Girls: A Questionnaire-Based Study in Turkey. J Clin Res Pediatr Endocrinol 2016. [Google Scholar]
- V Male. Menstrual changes after covid-19 vaccination. 2021. [Google Scholar]
