Introduction
Psoriasis is a chronic, noncommunicable, painful, disfiguring and disabling hyperproliferative disease with increased rate of epidermal turnover, for which there is no cure, presenting without a single, typical clinical picture.1 The commonest form of psoriasis is plaque psoriasis in which patients may have sharply circumscribed, round-oval, or coin-sized plaques. The lesions may initially begin as erythematous macules (flat and <1 cm) or papules, extend peripherally, and coalesce to form plaques of one to several centimetres in diameter. Guttate psoriasis, describes the acute onset of a myriad of small, 2–10 mm diameter lesions of psoriasis. Inverse Psoriasis affecting the flexures, particularly inframammary, perineal, and axillary, is distinct morphologically from traditional plaques elsewhere on the trunk and limbs, Generalised pustular psoriasis is rare and represents active, unstable disease. Psoriatic nail disease: Fingernails are more commonly affected than toenails. The commonest finding is small pits in the nail plate, resulting from defective nail formation in the proximal portion of the nail matrix.2
Prevalence of psoriasis ranges between 0,5 to 4.7%. It can occur at any age. Symptoms often begins between ages 15 and 25, but is most common in the age group 50–69. Children account for 4.4% patients Male to female ratio is 1:2.5 .Most of the data is derived from hospital based studies. 3 25% of people with psoriasis could be classified as having moderate to severe psoriasis. Nearly 30% have arthritic problems. In people who have skin psoriasis 10=55 have psoriasis of nails. There is genetic susceptibility to psoriasis, 40% of patients have positive family history. HLA-Cw6 is implicated gene. Up to 60% of patients describe stress as being a key “exacerbator” or trigger of their disease. 4 Precipitating factors for psoriasis are stress (upregulation of catecholamines), 2.5 times more in smokers, higher prolactin levels, Obesity (adipocyte proliferation), infection , trauma and drugs like B -blockers, lithium and antimalarials often show correlation.
Cytokines storm and Psoriasis
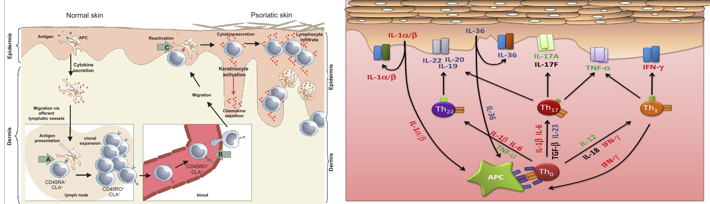
The clinical features of psoriasis, such as the hyperproliferation of keratinocytes, increased neovascularization and inflammation, and that by determining which cytokines played a central role in the disease process, interesting therapeutic targets could be identified (Figure 2a}. 5 In the time since, drugs targeting tumor necrosis factor (TNF)-α, interleukin (IL)-12/23, IL-17, IL-22, IL-23, granulocyte monocyte-colony stimulating factor (GM-CSF), as well as inhibitors of the Janus kinases (JAK1/2/3) downstream of a number of cytokine receptors. (Figure 2b)
The importance of type I IFNs in the triggering of psoriasis is strengthened by the well-documented induction of new-onset psoriasis or exacerbation of pre-existing psoriasis during treatment with IFN-α2. Psoriasis lesions have long been known to contain elevated levels of IFN-γ, mostly secreted by skin T cells. IFN-γ contributes to the cytokine storm in psoriasis by, aiding and abetting other cytokines, in particular, IL-17, as well as playing a role in priming APCs, which drives IL-1/IL-23 production to augment Th17 responses Figure 3.5
Using Topical Approach
First line management of adult mild-to-moderate adult plaque psoriasis is with topical treatment, including vitamin D analogues and topical corticosteroids. Topical therapies are indicated for patients whose affected area is < 10% of the body surface area. Topical vitamin D analogues and topical steroids are both widely used topical treatments. Calcipotriol, a vitamin D3 analog, acts not only to inhibit cell proliferation and enhance cell differentiation in the skin of patients with psoriasis, but also appears to have effects on immunologic markers that are thought to play a role in the etiology of the disease. Lower-potency corticosteroids are particularly recommended to apply on the face, groin, axillary areas, and in infants and children, whereas mid- and higher-potency corticosteroids are commonly used as initial therapy on all other areas in adults. Superpotent corticosteroids are mainly used for stubborn, cutaneous plaques or lesions on the palms, soles, and/or scalp.
Topical corticosteroids can aid in the treatment of psoriasis by:
Reducing inflammation
Slowing the hyperproduction of skin cells
Reducing the appearance of psoriatic skin lesions
Alleviating itching and discomfort
Aiding the shedding of scaly skin
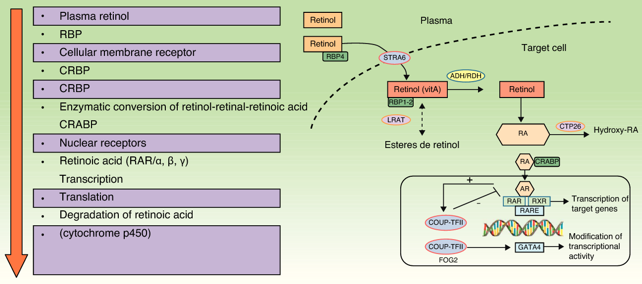
Systemic retinoids are known to have immunosuppressive and anti-inflammatory activity and to modulate epidermal proliferation and differentiation. Tazarotene and acitretin are the only retinoids that are available in both topical and systemic formulations
Acitretin is an oral retinoid used in the treatment of extensive and severe refractory forms of psoriasis and Pustulous psoriasis of the hands and feet. Because of the potential for problems and severe side effects it is generally used in only very severe cases of psoriasis that have been unresponsive to other treatments. Acitretin is highly teratogenic and noted for the possibility of severe birth defects.
Tazarotene is a third-generation topical retinoid available as a cream, gel, and foam. Tazarotene properties are similar to that of Vitamin. In the treatment of psoriasis, it may be used in a combination with a corticosteroid cream or ointment, calcipotriol or phototherapy.
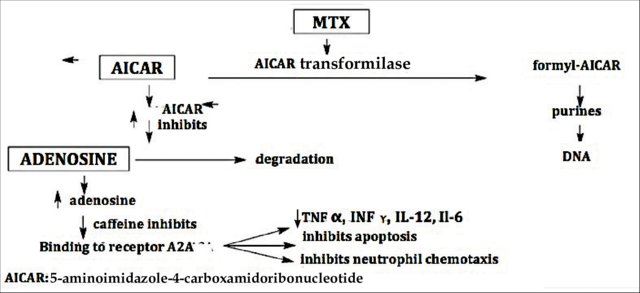
Methotexate orally helps psoriasis by decreasing the production of skin cells and suppressing inflammation. It may also slow the progression of psoriatic arthritis in some people. Methotrexate is generally well tolerated in low doses. MTX is indicated as a systemic treatment in moderate to severe plaque psoriasis, psoriatic erythroderma, generalized pustular psoriasis, nail psoriasis, palmoplantar psoriasis, and, especially, in psoriatic arthritis. It is a good therapeutic choice for the treatment of psoriasis that has failed to respond to topical therapies, acitretin, broadband and narrowband psoralen-UV-A (PUVA) and UV-B, or when these therapies are unavailable or have been rejected by the patient.5, 6
Cyclosporine suppresses the immune system and is similar to methotrexate in effectiveness. It induces immunosuppression through preventing down-stream T-cell activation. It inhibits the activation of Nuclear factor of activated T-cells (NFAT) & further inhibition of gene transcription of IL-2 by T cells. Cyclosporine can effectively treat severe and disabling psoriasis, and it works quickly. In clinical trials, 80% to 90% of patients who received cyclosporine for 12 to 16 weeks had rapid improvement.7
Mofetil (MMF) has higher efficiency than methotrexate and cyclosporine in the treatment of psoriasis. MMF requires monitoring as it varied side effects. MMF may represent a good alternative for the treatment of psoriasis in patients who are unable to take MTX or other available drugs due to contraindication or toxicity.
Tacrolimus mode of action is inhibiting the production of interleukin-2. Topical tacrolimus is particularly effective for inverse psoriasis, which is likely to be due to the reduced level of induration seen in these psoriatic lesions, which allows greater skin penetrance, compared with hyperkeratotic plaques of psoriasis on the body. It is also notable that the areas affected by inverse psoriasis are more susceptible to adverse effects of topical corticosteroid therapy. Topical tacrolimus is prescribed off-license for the treatment of psoriasis,
Dimethylfumarate and/or monomethylfumarate: it is thought to involve a reduction in the production of inflammatory cytokines, promoting apoptosis, inhibiting keratinocyte proliferation, reducing expression of adhesion molecules and lowering inflammatory infiltrate in psoriatic plaques. Dimethyl fumarate is used for the treatment of moderate to severe plaque psoriasis in adults when systemic treatment is indicated. NICE’s technology appraisal concluded that dimethyl fumarate improves severe psoriasis more than placebo but indirect comparisons suggest it is less effective than systemic biological therapies and apremilast
Anthralin (Dithranol) reduces keratinocyte proliferation, prevents T cell activation, and restores cell differentiation, probably through mitochondrial dysfunction, It cannot be used on the face or genitalia. Short contact anthralin therapy (SCAT) is advocated for the treatment of localized, scaly plaques of psoriasis. Side effects include a local burning sensation and irritation
Tar preparations are helpful to remove loose scales of the patches of psoriasis. It is the first-line treatment for scalp, hand, and foot psoriasis Tar preparations are available in the form of creams or ointments or shampoos. The mechanism of action of coal tar is not well understood. Postulated effects include a suppression of deoxyribonucleic acid (DNA) synthesis and subsequent inhibition of keratinocyte proliferation. Coal tar is thought to help correct the defect of differentiation in keratinocytes of patients with psoriasis.
Apremilast: is an orally administered, small molecule inhibitor of phosphodiesterase 4(PDE4). (Figure 4) .Apremilast 30 mg twice daily reduced the severity of moderate to severe plaque psoriasis in the phase 3 ESTEEM trials, as well as improving difficult-to-treat nail, scalp and palmoplantar psoriasis. [6 a,b}
Light therapy: UVB exposure reduces the rate of DNA synthesis. In addition, UVB radiation causes photoisomerization of trans-UCA to cis-UCA which has immunosuppressive effects.
Photochemotherapy or PUVA: has been found to be effective in psoriasis in well-conducted studies and randomized controlled trials. Psoralen derivative most widely used in PUVA is 8MOP (methoxsalen). Psoralens enter the cells and intercalate between DNA base pairs. On exposure to UVA, psoralens absorb photons, become chemically activated and covalently bind to DNA base pairs forming crosslinks. The DNA crosslinks have antiproliferative, antiangiogenic, apoptotic, and immunosuppressive effects. It is a relatively safe modality for the treatment of psoriasis, which is a chronic disease with remissions and relapses. Indications of PUVA are:
Psoriasis involving >20% body surface area
Unresponsiveness to topical therapy
Localized psoriasis of hands and feet (hand/foot unit
Localized disease not responding to other modalities of therapy
The most significant complication of PUVA therapy for psoriasis is squamous cell cancer. Two carcinogenic components of the therapy include the nonionizing radiation of UVA light as well as the psoralen intercalation with DNA. Both processes negatively contribute to genome instability.8, 9, 10, 11, 12
Line of therapy in Psoriasis: Clinicians should be aware of the risk for nonadherence as they design and initiate treatment plans. Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor. 13
Usage of Biologicals in Psoriasis
The ideal therapy for psoriasis is an efficacious, long-lasting agent devoid of acute or long-term adverse effects, with minimal monitoring requirements, and a dosing regimen that eases compliance. There is no cure for psoriasis. Biologic agent has been shown to induce periods of remission in patients with moderate-to-severe psoriasis. Clinicians must carefully screen patients to assess whether they are suitable candidates for biologic agents. Prior to initiating therapy with any biologic agent, appropriate screening studies include TB testing, complete blood count (CBC), hepatic function panel, hepatitis profile, and pregnancy testing as required.
Biologic agents for psoriasis have been proven to offer patients an effective alternative therapy for moderate-to-severe psoriasis.
The mechanistic design of each of the biologics is based on one of four general strategies:
Reduction of the pathogenic T-cells
Inhibition of T-cell activation
Cytokine mediated immune deviation from a T-helper (Th) type 1 to a Th2 response
Blocking the activity of inflammatory cytokines
Biological drugs are less toxic to the body and more effective than traditional therapies. Thus, they should improve the quality of life of patients with psoriasis. The long term side effect of the biologics is still being defined.14, 15
However, despite the high efficacy of these biologics, 20% to 30% of patients remain insufficient responders or non-responders. Improving the response rate of patients to treatment requires the appropriate selection of biologics.
Table 1
Indications of Biological Therapy in Psoriasis
Table 2
Indications to cease Biological Therapy in Psoriasis.
Table 3
Anti TNF Agents
Table 4
Table 5
Anti-interleukin
Table 6
Table 7
Anti TCell Strategies
Table 8
The network meta analysis of drugs used in Psoriasis have shown following16, 17, 18, 19, 20, 21, 22
Biologics can cause side effects, but they tend to be less severe. The most common side effects are minor irritation, redness, pain, or a reaction at the site of injection
The biologic treatments anti‐IL17, anti‐IL12/23, anti‐IL23, and anti‐TNF alpha showed significant superiority compared with small molecules and the conventional systemic agents, with small molecules achieving better results than conventional systemic agents.
All of the anti‐IL17 agents and the anti‐IL23 guselkumab were significantly more effective than all of the anti‐TNF alpha agents except for certolizumab (i.e. infliximab, adalimumab, and etanercept), and the anti‐IL12/23 ustekinumab was superior to the anti‐TNF alpha etanercept.
When compared with placebo, in order of highest efficacy, the following biological agents are the best choices: ixekizumab (high‐certainty evidence), secukinumab (high‐certainty evidence), brodalumab (moderate‐certainty evidence), guselkumab (moderate‐certainty evidence), certolizumab (moderate‐certainty evidence), and ustekinumab (high‐certainty evidence).
Tofacitinib was superior to methotrexate, and no difference was shown between the other small molecules and the conventional drugs. 23
Risankizumab, an anti‐interleukin‐23 monoclonal antibody, achieved significantly (P < 0.001) greater Psoriasis Area and Severity Index (PASI) and static Physician Global Assessment (sPGA) clear or almost clear (0/1) responses than adalimumab in a phase III trial in patients with moderate‐to‐severe psoriasis. 24
Natural Treatment for Psoriasis
Psoriasis symptoms can be relieved by change in diet and life style. Fasting food period, low energy diet and vegetarian diets have improved psoriasis symptoms. In some treatments supplemented with fish oil shows a beneficial effect due to the presence of omega - 3 Fatty Acids and Vitamin E. Natural supplements or herbs used in psoriasis are supplement for healthy skin, they may augment actions of drugs used in psoriasis.25
Table 9
Psoriasis algorithm recommended in Chronic Plaque Psoriasis > 5 % BSA without Psoriatic arthritis (Figure 12) 26:
Combination Therapy: Combination, rotational, and sequential approaches are often more effective and safer than single-agent therapy. Synergistic enhancement is seen with most paired combinations of the four major therapies: acitretin, phototherapy (ultraviolet B/psoralen plus ultraviolet A), cyclosporine, and methotrexate. The new biologic agents may prove to be important adjuncts in combination/rotational/sequential approaches. The need for combination therapy in patients with moderate-to-severe psoriasis is obvious, and combined treatment with retinoid and phototherapy is the only well-documented combination regimen for this disease. Severe cases, however, may warrant the use of short term combination therapy with a biological agent and phototherapy or a cytostatic in order to achieve remission, followed by maintenance therapy with biological monotherapy. 27, 28
Conclusion
Nearly half of individuals with psoriasis over the age of 65. Psoriasis generally does not affect survival but has significant detrimental effect on quality of life (QOL). The primary cause of psoriasis remains unknown. Abnormal epidermal cell kinetics and abnormal activation of immune mechanisms are thought to be the major contributors. The underlying immunopathology of psoriasis is correlated with the altered regulation of various cytokines such as TNFa, IL-23 and IL-17. The available therapies only relieve the symptoms. The choice of treatment is absolutely based on the type and severity of the disease. In patients who do not respond adequately to traditional topical treatments, oral and systemic agents are prescribed. Psoriasis is also associated with an array of important medical and psychiatric comorbidities that require timely therapy to improve long-term outcomes. The role of insulin resistance in the pathogenesis of psoriasis is under investigation.